Technology
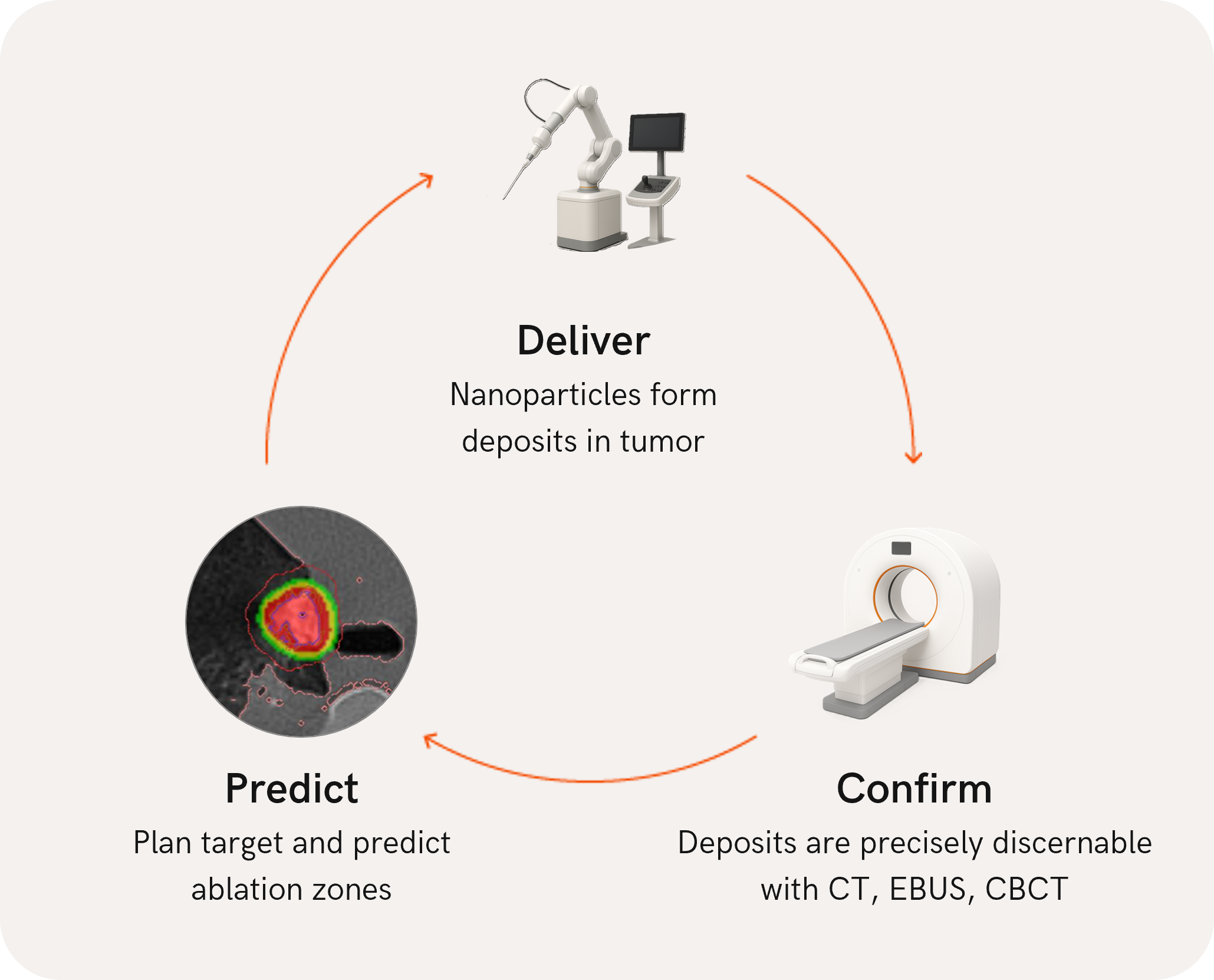
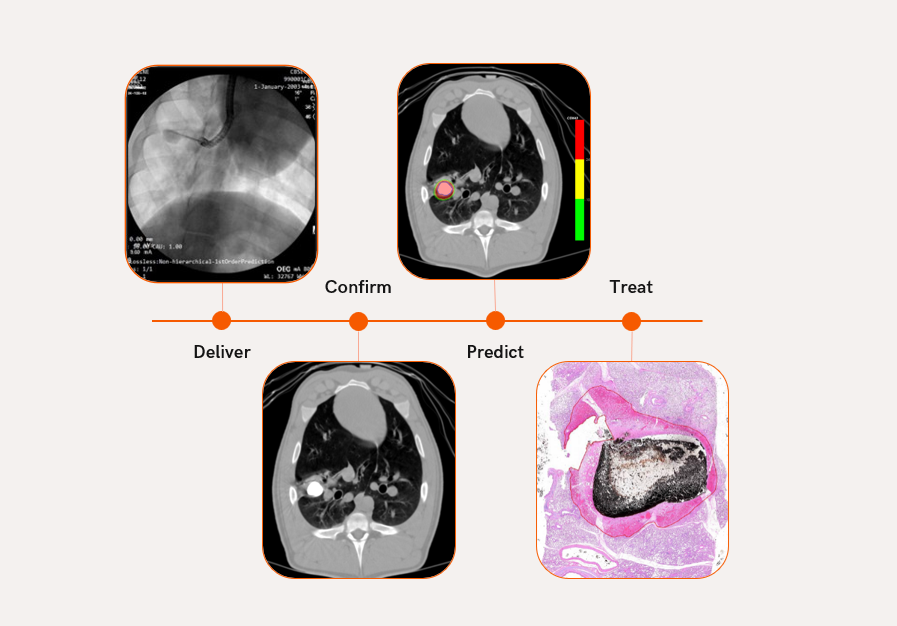
Confirm before treating: Next-level predictive tissue ablation using a clinically proven 2-step image guided therapy workflow
Advantages
Nanotherm is developing a thermal ablation method to treat solid tumors: Targeted thermal ablation enabled by nanoparticle activation.
Simple clinical workflow
Treatment with the Nanotherm technology can be done one day. Our vision is to diagnose and treat patients in the same day.
Insensitive to motion
During treatment, patients breathe, their hearts beat, and they move. A tumor is literally a moving target. Nanotherm allows for stable, controlled heating over time due to contact-less energy delivery, making inaccurate real-time tumor tracking unnecessary.
Tailored to target shape
Treatment based on the individual tumor volume and localization. Nanoparticles delivered via universal 19G needle, as no antenna is needed to deliver energy, those technical restrictions are no longer a concern for the ablation zone shape.
Product Suite
The Nanotherm Technology has been demonstrated in over 200 patients.
Nanotherm Therapeutics in numbers
Demonstrated progress in developing and delivering advanced oncological therapies.
200+
Patients with over 1000 activations treated with NTTS
25+
Years of research, development, and clinical progress demonstrating long-term toxicology and stability.
03
Clinical centers conducting research
100
Patents – Strong intellectual property across key global markets
QMS
Quality Management System is ISO 13485:2016 conformal. Previously: MDR certified.

